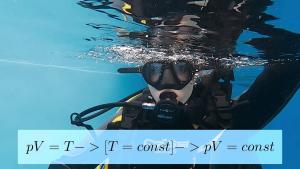Table of Contents
Lungs diver and the effect on them
Diving theory teaches that a diver fills the lungs by breathing compressed air into the underwater environment, and the volume of air at the time of lifting increases more than the lungs can hold as the ambient pressure decreases. Fortunately, lung barotrauma is not common, but it is possible if a person has breathed air from a balloon in an underwater environment and for some reason is holding his or her breath.
Emergency take - off procedures for divers
The Open Water Diver teaches the diving course "Controlled Emergency Swimming Ascent" (CESA), which is the correct emergency take-off procedure in case the diver runs out of air using the sound "AAAAA", with the regulator murmuring.
How to Take PADI CESA Skills in the Water |
1. Start with a neutral buoyancy, simulate the take-off position with your right hand over your head and your left hand on the deflator button.
2. Take a few deep breaths
3. When you are ready, take a final breath and start swimming slowly, exhaling continuously, making aaaaaaaaaaah sound.
4. Continue to swim slowly without touching the bottom or surface
5. Continue to exhale, creating this continuous sound
6. Swim at least 9 meters until CESA is complete
Boila-Mariota law
This process is described by Boila-Mariota's law for a closed system with a constant temperature. The pressure in this system varies inversely with the volume. pV = T → [T = const] → pV = const It is assumed that the temperature in the diver's lungs is constant, and as the pressure on the person increases as the water depth increases, the human lungs are also compressed, thus limiting their expansion. This problem is solved by breathing air through a balloon where it is compressed, thus equalizing the air pressure in the lungs.
Does the volume of air decrease when diving down?
With a glass you can see how the volume of air decreases when diving down, but increases when swimming up. It also happens to the human lungs when it starts to float up. According to the same law, as the ambient pressure decreases, the lungs expand. Like everything around us, the lungs have their limits of elasticity, so you can't hold your breath when swimming up! Because the remaining air must be expelled from the lungs, otherwise it may result in lung rupture. So also stand out over the water gradually! Thanks to all the creators of this video and especially to its author Margarita for putting ideas and videos into the training of divers.
Where to apply for diving in Latvia?
🤿😀 For advice or assistance, call a PADI Diver Instructor t. 220-77-202 (WhatsApp 220-77-202) for advice and scuba diving.